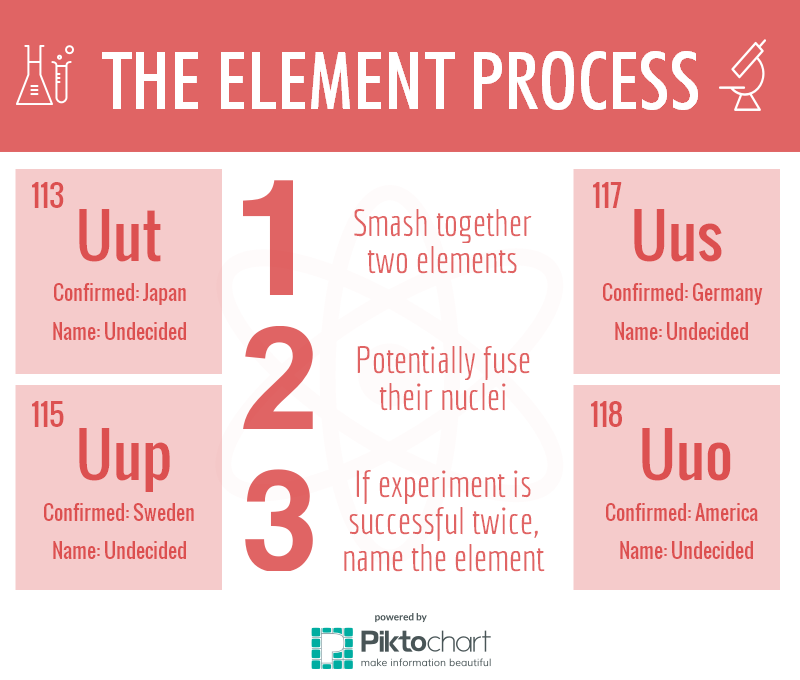
It takes two experiments to confirm the existence of an element, an action that will earn the element an official symbol and name. On Dec. 30, the International Union of Pure and Applied Chemistry verified elements 113, 115, 117 and 118 as discoveries and is now in the process of finalizing names and symbols for the synthetic elements. Now, chemists have a new goal: confirming the properties of the new elements.
“It would be nice to do something with the class in terms of them knowing that this is going on,” chemistry teacher Elizabeth McCracken said. “So maybe we can find an article and have [students] read it.”
According to the Royal Society of Chemistry’s website, new elements can be discovered in a multitude of ways; either through reactions with other elements such as carbon, the distillation of liquid air or even purely by accident. These four new elements in particular were discovered through the use of sub-atomic particles.
“The IUPAC’s job is to decide who really [found the element] first, and award that particular lab naming rights,” chemistry teacher Mary Murphey said. “If you can show from your lab experiments that you [found the new element] at a certain date and other people did it a month later, a year later, then it’s your discovery.”
Crashing nuclei into another nucleus creates a new, larger nucleus. As there is a lot of repulsion within subatomic particles, the new nucleus collapses and as a result, creates products consisting of elements.
“They might have just made a few atoms of this, they might have very small half-lives,” AP Chemistry teacher Kavita Gupta said. “But eventually maybe somebody can study them and figure out potential uses because everything you see on the [Periodic Table] has some use.”
Story by Shriya Deshpande and Issra Osman